Paper:
E-102 Early multi-center experience with the NeVa VS in the treatment of subarachnoid hemorrhage-related cerebral vasospasm
Authors:
Clemens M. Schirmer, Shahid Nimjee, Eric Savageau, Michael Chen, Ameer E. Hassan, Geisinger
Journal:
Journal of Neuro-Interventional Surgery
Background:
Cerebral vasospasm represents a formidable challenge in the aftermath of subarachnoid hemorrhage (SAH), significantly contributing to morbidity and mortality. This life-threatening and potentially reversible complication is conventionally addressed through percutaneous transluminal balloon angioplasty (PTA) or intra-arterial (IA) infusion of vasodilators, particularly when standard medical therapies prove ineffective. However, these endovascular interventions often fall short in efficacy or are marred by elevated complication rates, including vessel rupture and thromboembolic events. The persistent issue of frequent recurrence underscores a pressing unmet need in vasospasm management. In this context, the NeVa VS (Vesalio)—a patented, retrievable nitinol stent—emerges as a novel solution engineered explicitly for cerebral vasospasm post-aneurysmal rupture. Notably, it is the sole device approved for this application in the United States. This study delineates our initial clinical experiences utilizing the NeVa VS device.
Methods:
Our study encompassed more than 41 patients across six US centers, all treated with the NeVa VS device. Of 17 cases analyzed to date, all were confronting refractory symptomatic cerebral vasospasm after aSAH. The deployment of NeVa VS involved a 0.021’ or 0.027’ ID micro-catheter, positioning the stent across the affected vasospasm segment. Following a 4–5 minute interval, allowing the self-expanding nitinol structure to dilate the constricted vessel segment mechanically, the device was resheathed into the microcatheter and removed. We aimed to evaluate intraoperative efficacy, user experience, safety parameters, and preliminary outcomes in this patient cohort.
Outcomes:
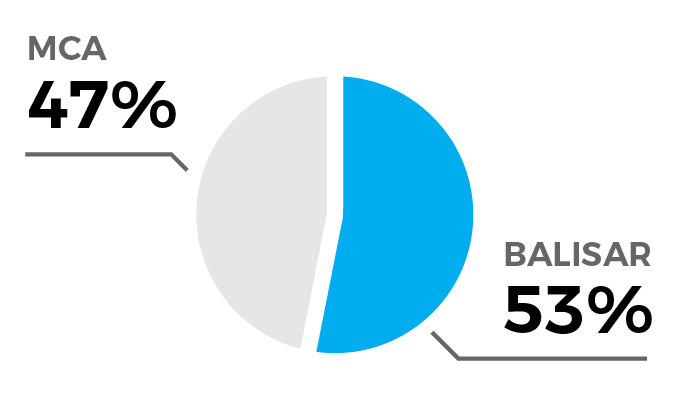
Comprehensive outcomes were ascertained for 17 out of the 41 patients. The median age was 45 years; 75% were female, and 81% of Hispanic/Latino ethnicity. The device was deployed, on average 14 days after the index hemorrhage, in total 18 times (1.1 per case) in the MCA (47%) or basilar artery (53%) for an average of 6.6 minutes. With one exception, all treatments were deemed successful (94%) with significant improvement in the degree of spasm from 84% pre- and 27% post-intervention, and no safety events occurred, in particular no strokes and intraprocedural ruptures. Two patients (12%) required additional treatment, on average 11.5 days after the first treatment. Parameters such as vessel caliber pre- and post-intervention, procedural metrics, and outcomes—including symptom relief, patient status changes, and adverse events—were meticulously analyzed.
Treatment Information:
17 vessels treated (8 MCA, 9 BA)
18 deployments (average of 1.06 per vessel)
Average deployment time: 6.6 min [range: 2-10 min]
(1.06 average) [1-2]
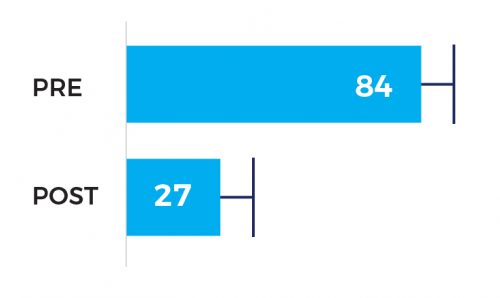
Degree of stenosis 84% pre vs 27% post
Overall Treatment Success:
1.
Treatment successful in 94% of patients (16/17)
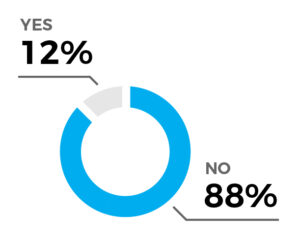
Further treatment required in 12% of patients (2/17)
Average interval to 2nd treatment: 11.5 days
2.
Significant improvement in the degree of spasm from 84% pre- and 27% post-intervention
3.
no safety events
no strokes
no intraprocedural ruptures
Conclusion:
In this preliminary multi-center exploration, the NeVa VS device demonstrates a 94% success rate in performing a safe and efficacious single-stage intervention to address severe stenosis secondary to vasospasm post-aSAH. The device’s ability to control vessel expansion and preserve distal flow offers a significant edge over traditional balloon angioplasty. It also exhibits a lower risk profile concerning vessel rupture, marking a significant advancement in neurointerventional therapy.